10 min
Development
Reducing Proton Therapy Range Uncertainty with Photon‑Counting CT
Proton therapy depends on extremely precise knowledge of how protons travel through human tissue.
Overview
Proton therapy depends on extremely precise knowledge of how protons travel through human tissue. Even small inaccuracies in tissue characterization can shift where the proton beam deposits its maximum dose (the Bragg peak), potentially under‑dosing tumors or over‑exposing nearby organs at risk.
This article summarizes and translates a recent scientific study that investigates how photon‑counting CT (PCCT) combined with vendor‑agnostic tissue characterization software (TissueXplorer) can reduce range uncertainty in proton therapy. Importantly, the work uses a virtual imaging framework developed at Duke University to compare dose distributions against a known ground truth in realistic head anatomy - something that is difficult to achieve experimentally.
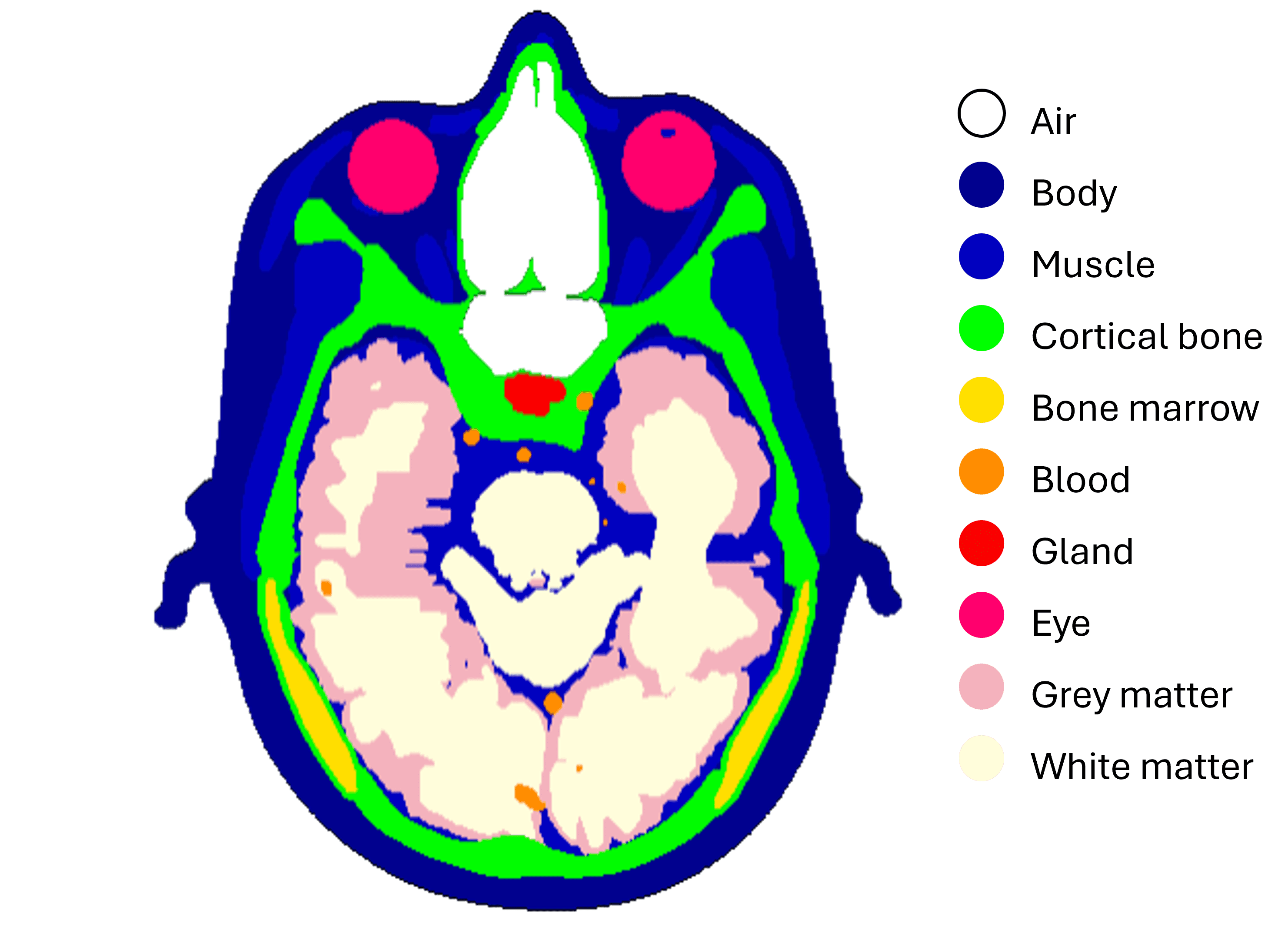
Why Range Uncertainty Matters in Proton Therapy
In clinical practice, proton range uncertainty is typically on the order of 3–3.5% of water‑equivalent path length. A major contributor to this uncertainty is how conventional CT scanners convert Hounsfield Units (HU) into stopping power ratio (SPR) using stoichiometric calibration curves.
Key limitations of the conventional approach include:
Scanner‑ and center‑specific calibration protocols
Sensitivity to image noise and artifacts
Limited ability to distinguish between tissues with similar HU but different elemental composition
Reducing this uncertainty could help clinicians to:
Shrink safety margins
Improve dose conformity
Better protect organs at risk (OARs)
Photon‑Counting CT: A Step Forward
Photon‑counting CT is the latest evolution in CT imaging. Unlike conventional energy‑integrating detectors, PCCT:
Separates X‑ray photons by energy
Produces lower‑noise images
Enables spectral information to be used directly for tissue characterization
These properties make PCCT particularly attractive for proton therapy planning, where accurate material characterization is critical.
Virtual Imaging as a Validation Tool
One of the most novel aspects of this study is the use of virtual imaging trials instead of purely physical phantom measurements.
The Virtual Framework
DukeSim: A validated CT simulator capable of modeling both conventional and photon‑counting CT scanners
XCAT anthropomorphic head phantom: A computational model with known tissue compositions and densities
Ground‑truth SPR maps: Calculated directly from elemental composition using the Bethe–Bloch equation
This approach enables a voxel‑by‑voxel comparison between:
Ground‑truth dose distributions
Dose distributions recalculated from CT‑derived SPR maps
TissueXplorer: Vendor‑Agnostic SPR Estimation
TissueXplorer is a prototype software system designed to extract accurate tissue properties from spectral CT data.
Instead of relying on HU‑to‑density calibration curves, TissueXplorer:
Uses virtual monochromatic images
Applies dictionary‑based tissue classification
Estimates voxel‑wise chemical composition, density, and SPR directly
This makes the method:
Vendor‑agnostic
Less dependent on scanner‑specific calibration
Compatible with existing treatment planning workflows
Treatment Planning Setup
To evaluate clinical impact, proton treatment plans were created for two challenging scenarios:
Nasal tumor
Brain tumor (near the brainstem)
Key parameters:
Single lateral beam
2 Gy (RBE) single‑fraction dose
Robust optimization applied
Three plans were compared:
Ground‑truth plan (known SPR values)
Single‑energy PCCT plan using stoichiometric calibration
PCCT plan using TissueXplorer SPR maps
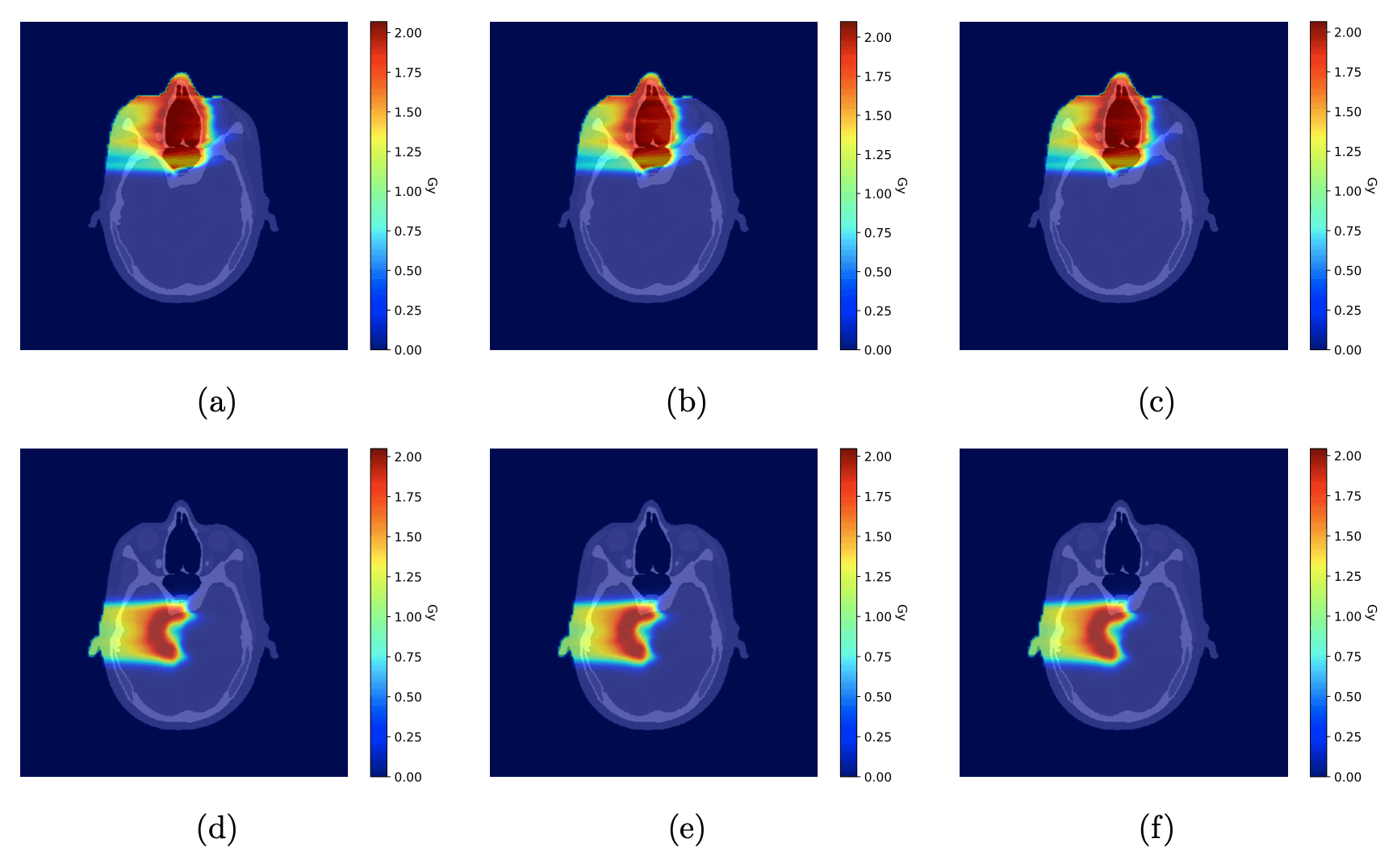
(a, d) Ground-truth dose distributions, calculated using known tissue compositions, for the nasal tumor (a) and brain tumor (d). (b, e) Dose distributions recalculated using a conventional single-energy CT stoichiometric calibration for the nasal tumor (b) and brain tumor (e). (c, f) Dose distributions recalculated using TissueXplorer, which derives tissue properties from spectral CT data, for the nasal tumor (c) and brain tumor (f). All plans deliver a single 2 Gy (RBE) fraction. Colors indicate dose in Gy.
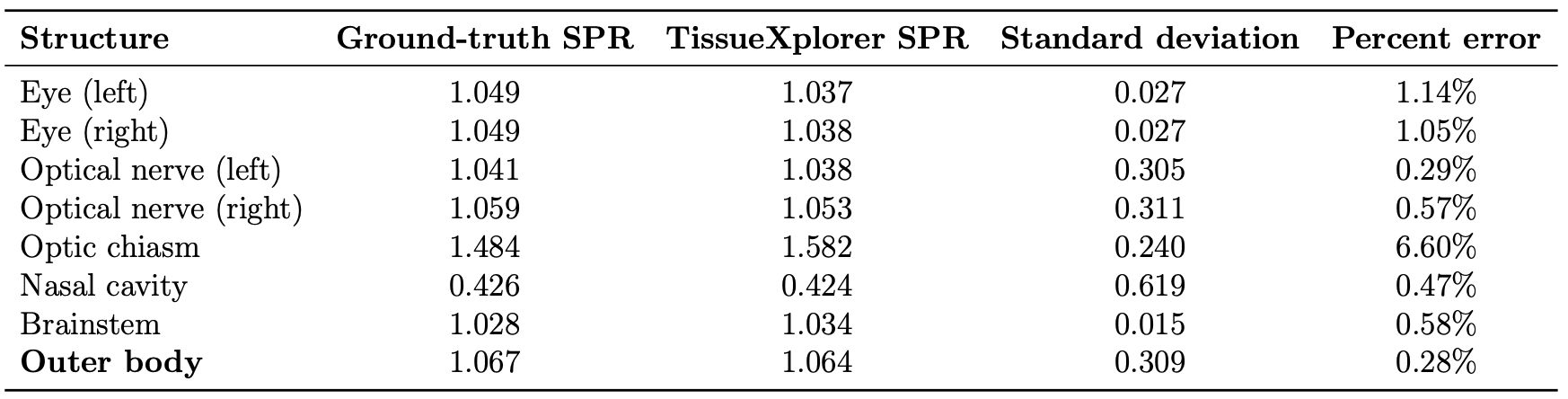
SPR Accuracy
Across all tissues in the scanned head volume:
Mean SPR error with TissueXplorer: 0.28%
This represents a substantial improvement over conventional calibration approaches.
Dose Distribution
The largest dose discrepancies occurred near the distal edge of the proton beam, where Bragg peak position is most sensitive to SPR errors.
Plans using TissueXplorer closely matched the ground‑truth dose distribution.
Conventional single‑energy calibration showed larger under‑ and over‑dosing, particularly near critical structures such as the optic nerves.
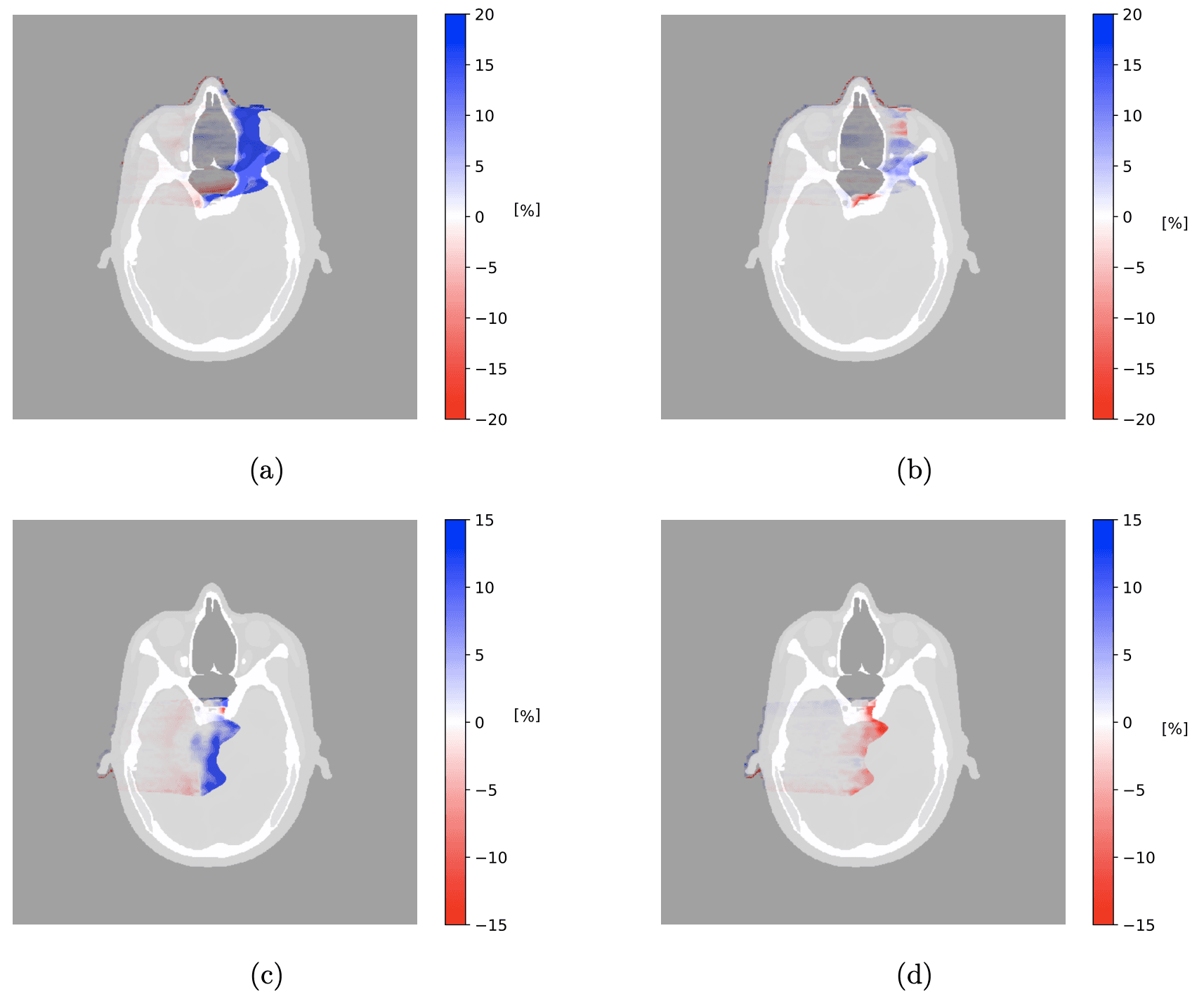
Difference in dose distribution between ground-truth plan and recalculated single-energy photon-counting CT plan for (a) nasal tumor CTV and (c) brain tumor CTV, and ground-truth plan and recalculated PCCT plan for (b) nasal tumor CTV and (d) brain tumor CTV. The scale bar shows percent differences.
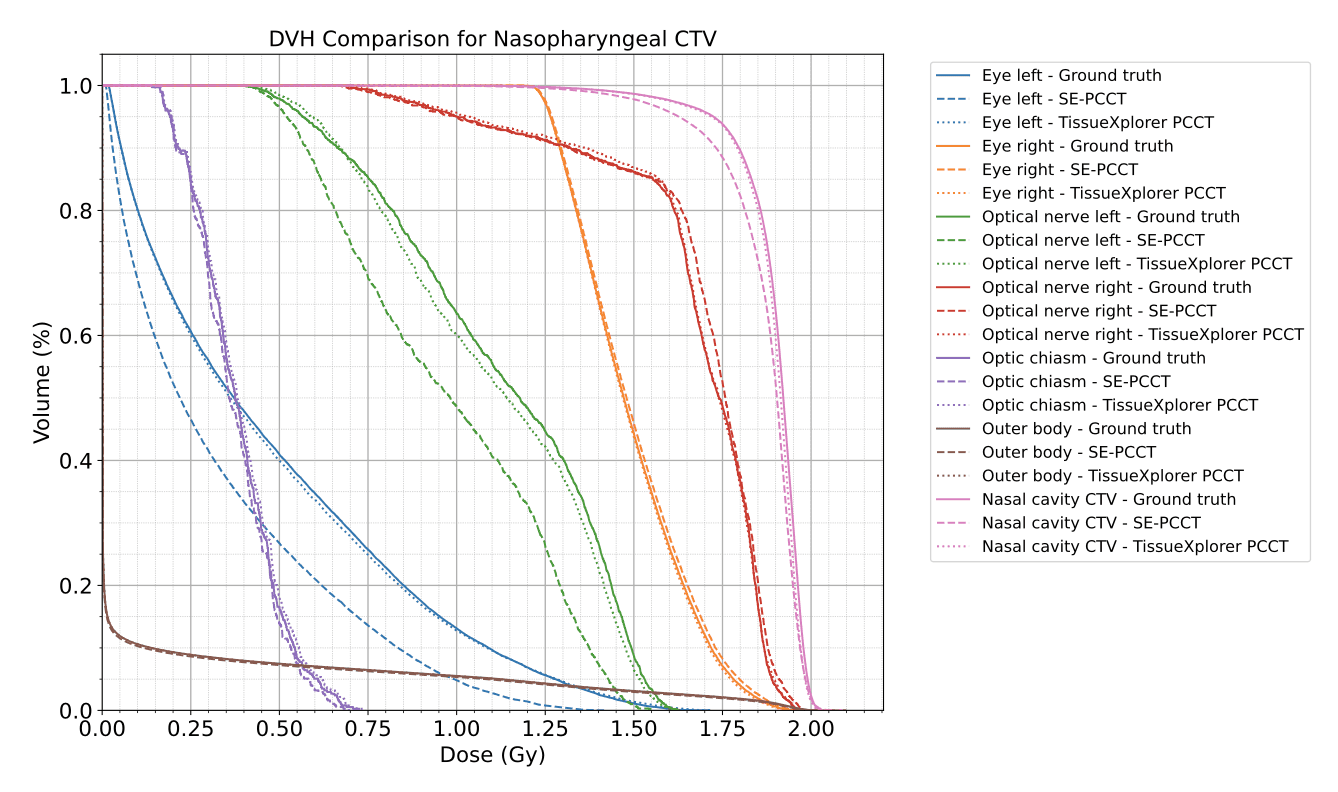
Dose‑Volume Histograms (DVHs)
TissueXplorer‑based plans reproduced target and OAR DVHs almost identically to ground truth.
Conventional calibration underestimated the dose to the clinical target volume in the nasal tumor case.
Clinical Implications
More accurate SPR estimation could be translated into:
Reduced range uncertainty margins
Improved sparing of organs at risk
Greater confidence in highly conformal proton plans
The study suggests that combining PCCT with spectral‑aware software such as TissueXplorer could enable safer and more precise proton therapy treatments.
Limitations and Future Work
While promising, this study was based on:
Single‑fraction treatments
Single beam angle per case
Future investigations will explore:
Multi‑beam clinical scenarios
Population‑based anatomical variability
The impact of tissue composition variability on SPR estimation
Conclusion
Virtual imaging provides a powerful new way to validate CT‑to‑SPR conversion methods in proton therapy. In this study, photon‑counting CT combined with vendor‑agnostic spectral tissue characterization produced dose distributions significantly closer to ground truth than conventional stoichiometric calibration.
These findings support the potential of PCCT‑based workflows to reduce range uncertainty and improve clinical outcomes in proton therapy.


